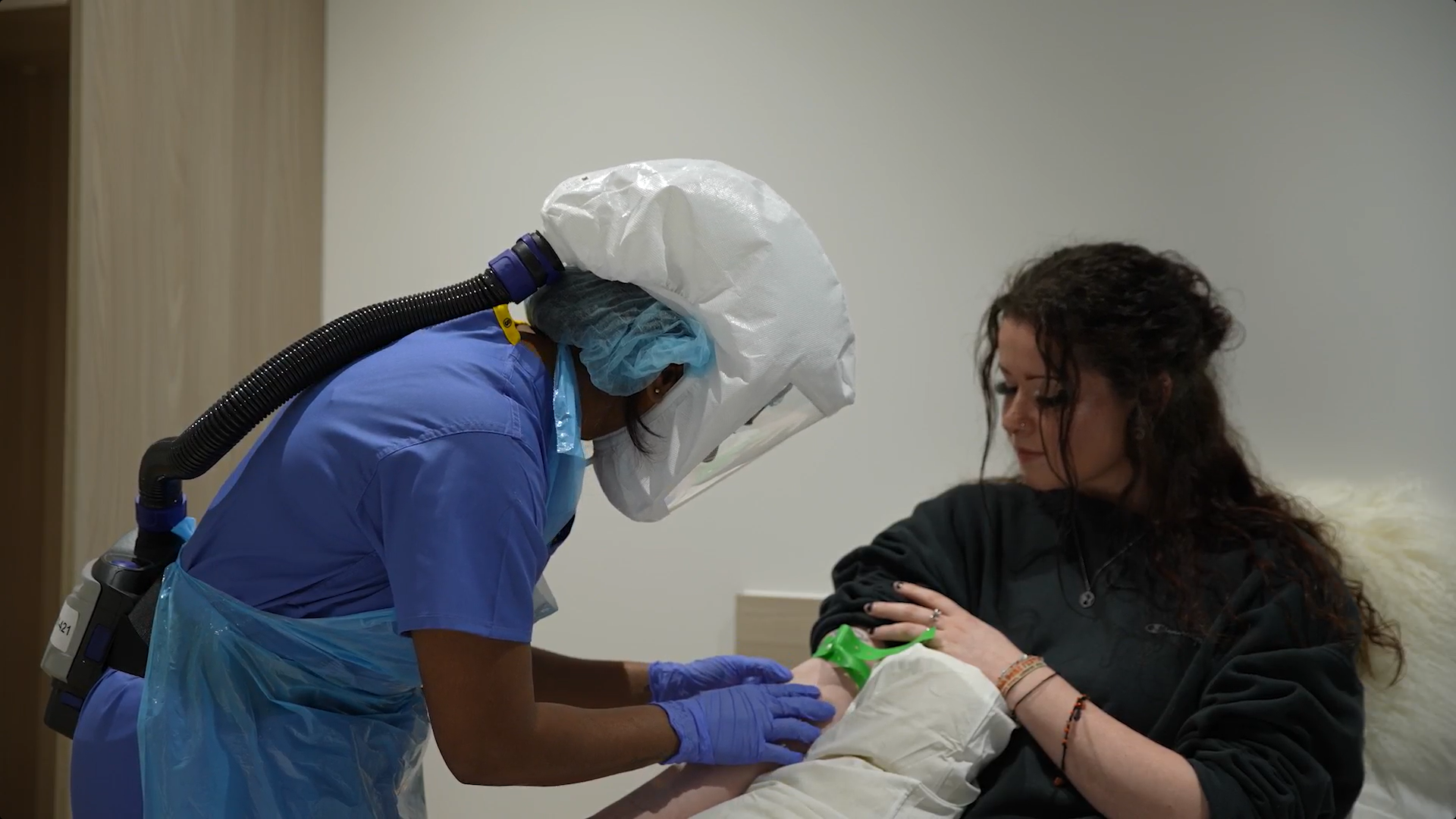
Participating in a clinical trial can be exciting, as you have an opportunity to make a mark on the medical industry, but at the same time, it can also be a confusing time and you may be wondering ‘can clinical trials make me ill?’. In some cases, such as a phase one trial, the true risks and side effects of the interventions being studied still need to be established.
This guide will help you better understand what you can expect when it comes to the potential health impacts of participating in a clinical trial. We’ll take a look at the landscape of clinical trials, consider the reality of the risks and also review the processes that staff use to screen for qualifying participants, along with how you can volunteer for a clinical trial.
The landscape of clinical trials
When it comes to asking questions like ‘do clinical trials cause illness?’, it’s important to understand the landscape of these studies.
Clinical trials are key to determining if a new treatment is effective and safe in people and can aid researchers in learning more about how the treatment can affect patients.
There are governing bodies and authorities that oversee the initiation and overall running of clinical trials. These governing bodies set numerous standards that clinical trials, including all the staff members involved in the study, need to follow.
One of the primary objectives of these governing bodies and the regulations they implement is to ensure the safety of clinical trials. They also cover ethical considerations and outline important factors that researchers need to take into account when screening candidates for participation in the study.
Clinical trials vs. reality
Understanding the safety of these studies involves learning more about the clinical trial risk, but also ensuring you can differentiate between misconceptions and reality.
There are many people who feel unsafe with the idea of using experimental treatments on themselves. There’s the concern for their own safety, not only in the short-run but also in the long-term. When researchers aren’t completely sure how a drug will affect patients, they use clinical trials to collect more data.
However, it’s important to note that these researchers tend to take as many precautions as possible. They do thorough research on the potential use of the new intervention before they even announce a clinical trial.
You’ll also notice that the interventions are often started off at a very minimal dose, which further contributes to the safety of the participants in the study. It’s safer to start with the smallest dose possible, and then to gradually work up to a larger dose. This helps to ensure that researchers can detect side effects, and when this happens, reduce your dosage to ensure they don’t put your health at risk.
The reality of the situation is that there are clinical trial risks that you’ll face when you decide to participate in one. However, the drugs and interventions used in these trials have already undergone a lot of research. When you take a look at statistics, you’ll notice that the actual rate of side effects from clinical trials is much lower than many people might think.
According to research, one important aspect to keep in mind is the fact that the risk of adverse reactions can significantly increase when a participant takes multiple medications. If you already use prescription drugs, then it’s important to mention this to those conducting the clinical trial. This will give them more details about potential interactions that could occur between the pharmaceutical drugs you’re taking and the interventions that they’ll be using as part of the study.
Understanding informed consent
Something that’s really important in any kind of clinical trial is informed consent. When we initiate a clinical study, we always ensure participants have access to comprehensive details about the trial and the interventions that will be used.
It’s important to understand that this is also part of the process to make clinical trials safer for potential participants. When it comes to the informed consent part of the trial, someone will sit down with you and explain to you what you can expect from the study. You’ll also receive documentation and details about the consent form that you’re required to sign.
You should take a close look at all of the information that’s presented to you as part of the informed consent process. This will help you understand the risks and how safe the clinical trial is. Use this as an opportunity to also ask any questions you may still have.
The rigorous screening process
When you show interest in being part of a clinical trial, then there are several processes you need to go through. The organisers involved with these trials won’t just accept anyone.
There’s a screening process that needs to be completed when you sign up to participate in a trial. This screening process is rigorous and involves several tests, questions and other elements. This can include a full medical history review, physical examination and blood and urine tests. The screening process helps to ensure that researchers are able to choose only individuals who will be a good fit for that phase of the clinical trial, while also considering the safety of the participants.
Since the screening process is so rigorous, it can make a big difference when it comes to minimising the risk of side effects and dangers throughout the study period. The researchers can rule out participants with specific conditions that might lead to experiencing a negative reaction to the drugs or devices that will be used in the trial, for example.
How to volunteer for a clinical trial
There are some risks involved with clinical trials, especially during the earlier phases. However, organisers who conduct these trials have to ensure there are regulatory bodies, researchers and healthcare professionals on site. There are also strict processes that candidates need to go through before they are allowed to participate in the clinical trial. These are elements that increase the safety of these trials, but there are still some risks that remain. If you have questions about the potential risks of a clinical trial, contact FluCamp to get the answers you’re looking for. You can also take advantage of FluCamp’s website to help you find studies that you can volunteer for.