Flucamp is on a mission to improve
Our background
FluCamp is a leading clinical research recruitment organisation specialising in human challenge trials for respiratory illnesses such as the common cold, flu, RSV & COVID-19.
With over 25 years of experience and based in London and Manchester, FluCamp has been at the forefront of medical research recruitment, contributing to the development and evaluation of new treatments and vaccines that help people recover from these illnesses more effectively.
Our mission and values
At FluCamp, our mission is to advance scientific understanding, test innovative vaccines and antiviral treatments, and improve public health outcomes. We are committed to conducting high-quality clinical trials that uphold the highest standards of ethics and regulatory compliance.

Our Expertise in clinical trials
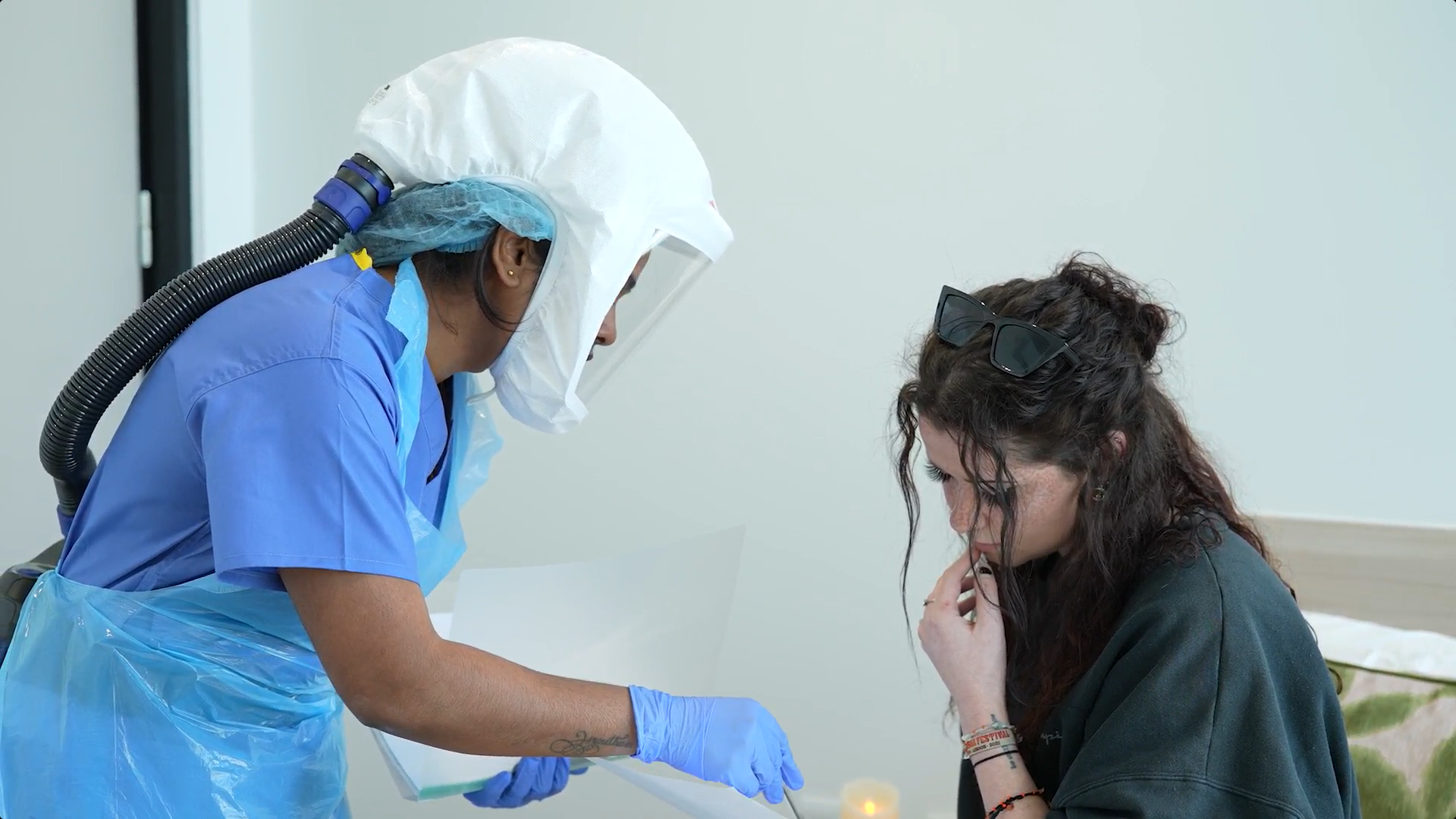
FluCamp has established itself as a global leader in recruiting for human challenge trials. Our state-of-the-art facilities, including purpose-built quarantine units and an experienced recruitment team, allow us to conduct controlled and safe clinical trials.

Commitment to Ethical Practices & Regulatory Compliance
At FluCamp, volunteer safety is our top priority. Our trials undergo rigorous review and approval from regulatory authorities such as: The Medicines and Healthcare products Regulatory Agency (MHRA).
We conduct comprehensive health screenings to ensure that only fit and healthy volunteers participate, minimising any potential risks. Our commitment to ethical research is unwavering, with continuous monitoring and adherence to strict guidelines throughout the trial process.

Volunteer experiences
Our volunteers are at the heart of everything we do. We provide a comfortable and supportive environment, ensuring their well-being at every stage of the trial. At the end of a clinical trial, volunteers are given a referral code that could earn them and a friend £250 if the code is used (at the point of application) and eligibility criteria are met.
Kyran, a previous FluCamp volunteer, described his experience, “I had a fantastic experience during my two-week stay at FluCamp. The staff were incredibly friendly, always happy to answer my questions and kept me well-informed throughout the trial. Their professionalism and warmth made the stay comfortable and stress-free.”

Apply now and get up to £4,400 in compensation
Your questions answered.
-
Volunteers joining FluCamp for a clinical trial stay with us on a residential basis in one of our purpose built rooms. Because it’s so important that the data we collect (from samples like blood and mucus ) is ‘clean’, volunteers must stay in quarantine conditions for the whole study. Additionally for this reason, our staff wear masks to comply with infection control guidelines. Please note, occasionally we may have studies available where no virus is administered and therefore residential may not be necessary.
-
We do contribute towards the costs volunteers incur when travelling to our clinics. For example, we will compensate volunteers £40 for their time and travel for attending their first pre-screening appointment to give a blood sample. This payment is processed via a link we send you in an email* approximately 3 days after your appointment. If you’re eligible to attend the second stage of screening we do assist further (£70).
*Please note this email comes from a company called Hyperwallet and not FluCamp.
-
For some studies which involve an initial screening, a blood sample may be required. This is a standard procedure which is unlikely to cause any problems, but on occasion it may be uncomfortable and there is a risk of minor bruising or phlebitis (inflammation of the vein). This is something that normally clears up itself with no further trouble. Our quarantine volunteers (the volunteers who have past two screening visits and who are invited to participate in the full trial) are given a diluted virus so it’s likely that they may experience some mild cold or flu like symptoms. We often then test a new medication that we would like to fight the virus. If we are testing a new medicine, volunteers may experience some minor side effects. Any side effects seen in the Phase I trials will be thoroughly discussed in the ICF and any questions can be directed to the physicians. Please note, before reaching FluCamp, the medication will have already been tested on humans and passed strict safety tests.
-
We understand that there may be some concerns when it comes to clinical trials. However, we have a very rigorous screening programme here at FluCamp, which means we only accept volunteers who we believe are fit and healthy enough to take part. We conduct in-depth health checks and you will not be selected if we do not believe you are fully healthy. This ensures volunteers who join a study are at minimal risk. The medication we use in our studies has already been tested on humans in accordance with relevant regulations. The virus is only administered in small doses and you will be monitored throughout the trial by our expert team in a safe environment.
-
Taking part in a clinical trial can offer many benefits, and we know our volunteers sign up for various reasons. Our testimonials show just some of the motivations for people to sign up. Whether studying, working, or taking a break from both, FluCamp gives volunteers the benefit of being cared for in every regard whilst having plenty of ‘me-time’. FluCampers also choose to stay with us because they like to use the compensation reward towards travelling, a house move, or just for a financial boost.
We run clinical trials because we are dedicated to finding a cure for viruses like the flu. We welcome all fit and healthy volunteers, and appreciate what they are doing to aid our work towards finding a cure!









