Our Covid-19 Human Clinical trial
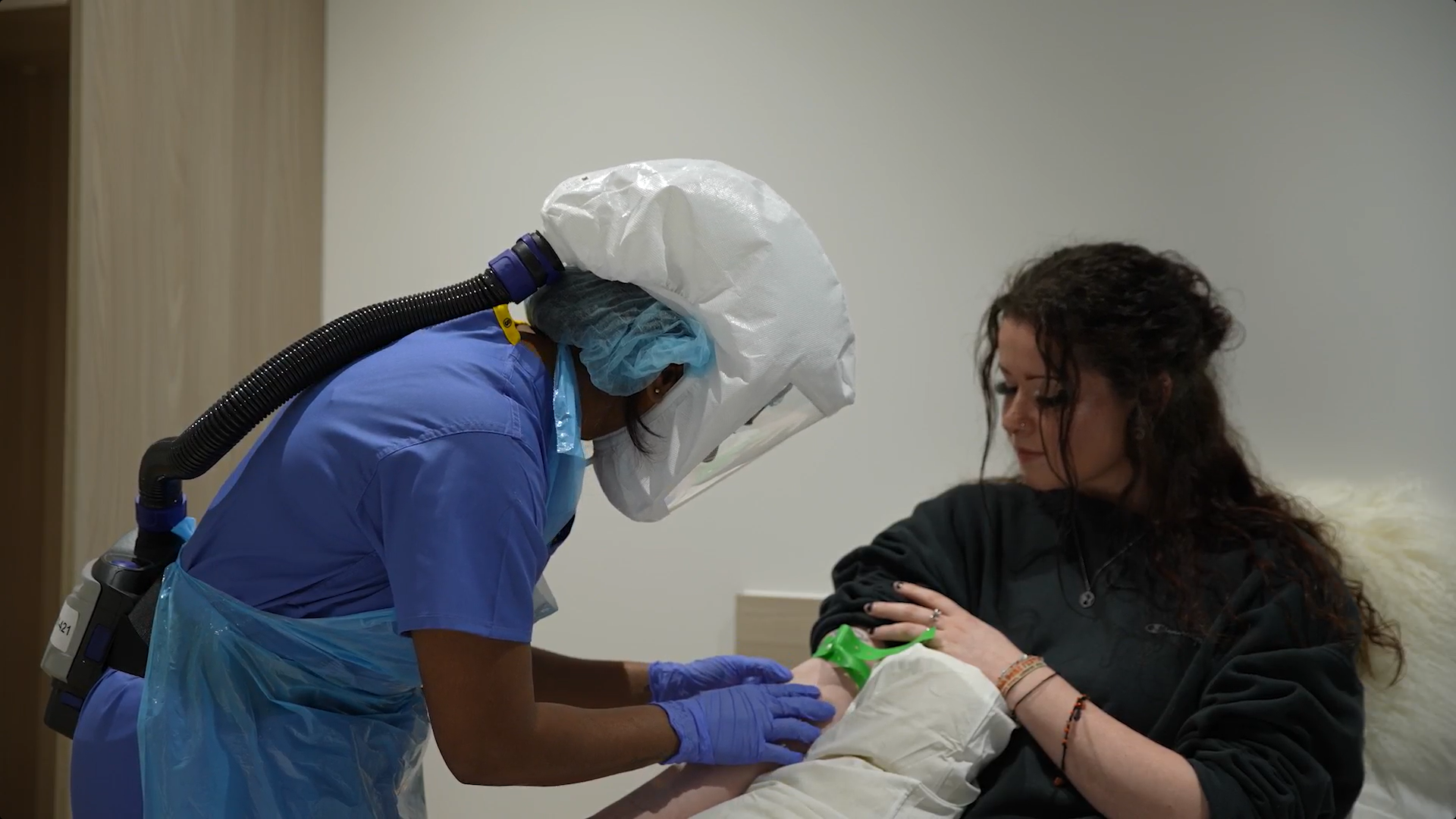
FluCamps’ parent company hVIVO recently published results for the world’s first COVID-19 human challenge trial, World’s first COVID-19 Characterisation Study (investis.com), which ran in Spring of 2021. FluCamp recruited 36 volunteers for this trial under the title of the UK Covid Challenge. The study was performed during the height of the corona virus pandemic and volunteers were based in the Royal Free London hospital.
The aims of the study included identifying an appropriate dose size of COVID-19 that leads to a safe and reliable infection. All volunteers were unvaccinated volunteers with no prior SARS-CoV-2 infection. We are excited to share the summary of these results here with all of you.
About the human challenge study
COVID-19 is the infamous virus that has plagued the globe for the past two years. It is a highly infectious respiratory disease that typically causes mild or asymptomatic in younger people but leads to serious infection and complications in those in at risk groups, including the elderly and immune-compromised. Recognising the potential benefits of COVID-19 human challenge study, the World Health Organization reviewed the pros and cons of balancing scientific and public health benefits while ensuring any risks to study participants were minimised and managed. But what is a human challenge study? A human challenge study is the model adapted in most of our trials at FluCamp. Human challenge studies involve giving participants a dose of active viral agent, usually to test novel treatments for the associated disease. These kinds of trials have been used for many diseases at FluCamp, including flu, RSV, and malaria. In our COVID-19 study we were only giving volunteers a dose of viral agent to be inline with the aims of the study. The strength goal behind this study was to make it possible for future challenge models to properly understand, the required dosage of virus for volunteers, the timing of exposure and the expected onset for symptoms. While anecdotal evidence was readily available from the public who have been infected, it is important for scientific studies to maximise understanding of the virus being used.
As this was the first study of its kind in the world, we were required to ensure volunteer safety was a top priority. Following extensive screening, participants were admitted to individual negative pressure rooms in an in-patient quarantine unit, with 24-hour medical monitoring. Volunteers remained with us for 14 days post infection appearing. Volunteers were given daily lateral flow tests to measure their viral load, the amount of virus in their system. Follow up visits were planned for up to 1 year to assess prolonged or ongoing symptoms, including smell disturbance.
What was found in the trial
Virus found in volunteers
- 18 volunteers (50%) became infected with viral load rising steeply and peaking at ~five days post-infection.
- No quantitative correlation was noted between viral load and symptoms.
COVID-19 Symptoms
- No serious symptoms were found in volunteers.
- Mild-to-moderate cold like symptoms were reported by 16 (88%) of 18 infected volunteers including a stuffy or runny nose, sneezing, and a sore throat. Some experienced headaches, muscle/joint aches, tiredness, and fever.
- Anosmia (lost or changed sense of smell) occurred in 72% of infected volunteers.
Virus detection
- Average time from first exposure to viral detection and early symptoms (incubation period) was 42 hours.
- Virus was detected earliest in the throat but at significantly greater levels in the nose.
- Virus detected in the throat on average after 40 hours.
- Virus detected in the nose on average after 58 hours.
- High levels of viable (infectious) virus were seen for approximately nine days post-inoculation, and up to a maximum of 12 days.
- Modelling using the study data indicated that regular asymptomatic lateral flow testing (“LFT”) would diagnose infection before 70-80% of infectious virus had been generated, thus if isolation was triggered would decrease community transmission to others.
Most importantly, no serious adverse events (SAEs) occurred, and the human challenge study model was shown to be safe and well tolerated in healthy young adults. With the characterisation study disease modelling data completed, and a COVID-19 Human Challenge Model now established.
Implications for Public Health
The results from this study have shown that a safe model for COVID-19 is possible and can be successful. This means that future scientists can look to “plug and play” with this study model, to test treatments to combat COVID-19.
The results show that the use of lateral flow tests is highly effective at detecting COVID-19, even before people begin to show symptoms. This supports the use of lateral flow testing to inform people of when to self-isolate and lead to limiting the transmission of the COVID-19 virus.
The study has also found that the incubation period, average of 42-hours, is a lot lower than expected, initially estimated at 5 to 6 days. Results also showed that while virus was detected significantly earlier in the throat, peak levels of virus were far higher in the nose, implying a potentially higher risk of viral shedding from the nose. This underlines the importance of proper facemask use to cover both the mouth and nose. Additionally, insights into the timeline of infection, with viable virus seen after nine days and 12 days for some, support the isolation periods advocated in most guidelines. A better understanding of incubation periods and viral agent load in different parts of the body can help us provide better legislation for infection control.
What our clinical trial shows us
We at FluCamp are very glad this study and feel a sense of pride to be involved in a ground-breaking study such as this. Even as the end of the pandemic appears on the horizon, we are still looking for better treatments and a further understanding of how to contain COVID should any further variants cause complications. Should you wish to help us develop treatments and garner a deeper understanding of other diseases please consider applying to FluCamp through the link below.